Risk Based Monitoring is about using the right people, clear processes and superior tools to run a better trial.
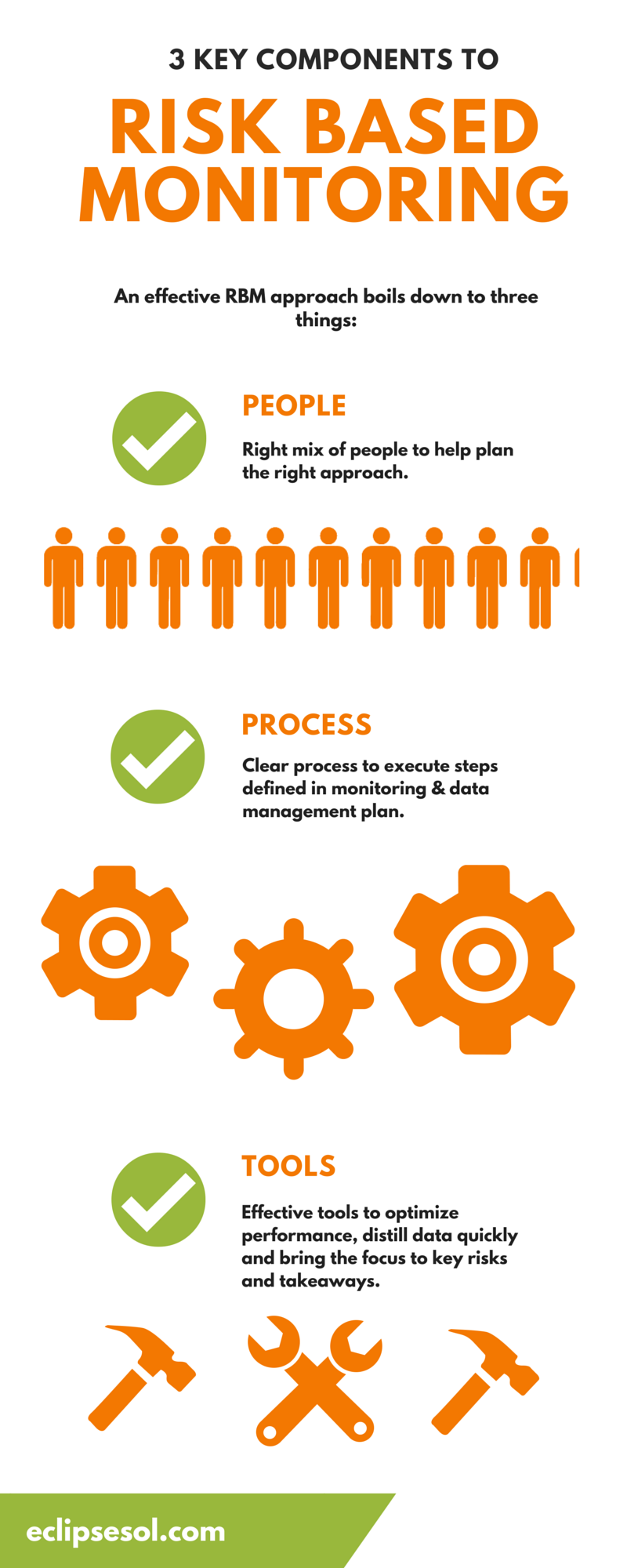
Risk Based Monitoring (RBM) is an increasingly popular approach to site monitoring and clinical data management that allocates resources based on risk and need, as opposed to delegating resources equally, regardless of each site’s unique challenges. An RBM approach aims to strategically use resources to ensure the safety of patients and the integrity of data. Benefits include superior data quality, lower costs, fewer errors and improved efficiency. An effective RBM approach boils down to three things: the right people, the right process and the right tools.
One of the starting points for any RBM strategy is ensuring you have the right mix of people to help plan your approach. Make sure every department is represented, including data management, clinical operations, biostatisticians and the medical and technology teams, and work together to define your plan. The philosophy behind RBM is to take a big picture approach to data monitoring and management and adapt as needed. The diverse skillset of your team will help you do just that.
2. Process
To execute the steps you defined in your monitoring and data management plan, you’ll need a clear process. Make sure you’ve spelled out everyone’s roles and responsibilities and hold team members accountable for their part in the process. Review and assess every step of the way and be prepared to make adjustments as needed. You’ll also need a strong communication plan to help encourage cross-department collaboration.
3. Tools
The clinical trial industry has seen incredible advancements in the technology it can use to support the effective execution of a study. Your tools should be able to clarify performance and distill data quickly so you can more easily hone in on what’s important and focus on key risks and takeaways. Effective software systems, such as Electronic Data Capture (EDC) systems help you make faster decisions and guide your course of action. There are a variety of Electronic Data Capture (EDC) and Clinical Trial Management Systems (CTMS) available that can help support a RBM approach. They’ll automate some of the steps your team would otherwise have to do manually, allowing people to work more efficiently. You’ll want to integrate your software systems as much as possible so different departments can easily access all data and benefit from all of the tools you’ve invested in.
Of course, the synergy between these three elements is paramount to a successful RBM approach. For example, you’ll need people who feel comfortable using these tools and a process for training them and communicating their findings across teams and platforms.
For more information on eclipse’s technology solutions, visit our Technology Page.