When and why to opt for Risk Based Monitoring, an increasingly popular approach to clinical trial site management
Site monitoring is one of the most costly factors of clinical trials. Traditionally, clinical trial teams have had to allocate equal resources to all study sites, regardless of risk. When adopting a Risk Based Monitoring approach, you work to identify the sites with the greatest risk and need and allocate resources accordingly. This intelligent strategy allows you to use your team’s time more efficiently, and often lowers the costs associated with site management. Risk Based Monitoring has become increasingly popular, as regulatory agencies in the U.S. and Europe have recently expressed support for the tactic.
The benefits of Risk Based MonitoringSource Data Verification (SDV) is expensive, but the conventional approach has been to perform 100 percent SDV. Literature states that SDV can consume about 25 percent of a trial budget. A lot of this cost goes to verifying data that are not critical. Focusing time and effort on non-critical data verification can affect the quality of review and source verification of critical data. CRAs can use their time more effectively if the monitoring is targeted, and they focus on quality rather than quantity.A quality by design approach which involves determining the risk indicators and a well 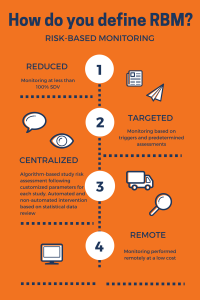 -designed monitoring plan will result in faster and smoother trial conduct and ultimately a successful regulatory submission. Additionally, integrated technology that provides a holistic view will aid in early trend analysis and help you make informed decisions early in the conduct of the trial.
-designed monitoring plan will result in faster and smoother trial conduct and ultimately a successful regulatory submission. Additionally, integrated technology that provides a holistic view will aid in early trend analysis and help you make informed decisions early in the conduct of the trial.
Some questions to ask when considering a Risk Based Monitoring solution:
- Does it offer real-time information on site performance and data quality?
- Does the solution provide ways to identify error ahead of time?
- Is the solution flexible enough to allow mid-study changes?
- Do you have a trained team to leverage the various tools that technology offers in the optimal way?
- How do the errors detected by monitors on a 100% SDV compare to those detected with reduced SDV?
Risk Based Monitoring may prove an optimal site management strategy for your next clinical trial, potentially saving you time and money. Ensure you understand the benefits of the approach and the details of the specific Risk Based Monitoring program you’re considering so you can make the right decision for your team.

