Importance of proper data collection and data management is manifold as it drives the outcome of a clinical trial. Clinical trial data should be accurate, timely and verifiable. Poor data is regarded as an oil spill by some, as it is difficult to clean up and involves time and money. Here are a few tips to ensure quality data collection and data management, thereby leading to sound clinical trials and better business.
Data Quality Tip # 1: Understand the purpose and objectives of the study.
The study protocol serves as a road map for understanding the context of the data being collected. A good understanding of the why’s and when’s of the data being collected is an important aspect pertaining to quality data.
Data Quality Tip # 2: Usage of appropriate reporting tools.
Creating reports for key data items like adverse events, screening and enrollment, Query status and aging, site performance, and their analysis will help address inconsistency/errors with data items that cannot be otherwise picked up using programmed edit checks.
Data Quality Tip # 3: Stay on top of missing data.
Define a completion timeline for missing data and follow up with the sites or CRA to get the missing data. This would avoid any undue pressure at a database lock.
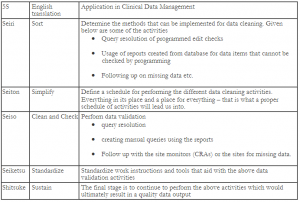
Here are some thoughts on how the 5-S Process of Total Quality Management (TQM) could be implemented for data cleaning:
Remember – Garbage in is Garbage out! A clean data input paves the way for a clean data output, which subsequently helps to transform the ‘data’ into useful ‘information’, ultimately leading the business to making well informed decisions.